Post Date:Mar-24-20
Overview of Novel Coronavirus Pneumonia
 Author: AcceGen R&D Team
Author: AcceGen R&D TeamA new outbreak of novel coronavirus (2019-nCoV) infected with pneumonia has been reported in China since mid-December 2019, and the WHO officially named it Corona Virus Disease 2019(COVID-19). The epidemic has gradually spread across the world, now the United State and some other countries and regions are facing a challenging situation. AcceGen R&D Team has summarized some published literature to have a systematic understanding of the new coronavirus, which is summarized as follows.
Discovery of the Corona Virus Disease 2019(COVID-19)
Medical institutions around South China Seafood City in Wuhan have successively treated multiple cases of pneumonia of unknown cause since mid-December 2019[1, 2]. Genome sequencing of the virus isolated from the patient’s lower respiratory tract on January 10, 2020, confirmed that this was a new type of coronavirus. Two days later, the World Health Organization (WHO) named it “2019 New Coronavirus (2019-nCoV)“[3].
2019-nCoV continues to spread globally and formed a large scale of the outbreak. Until now, tens of thousands of people have been infected with the 2019‐nCoV[4]. A novel coronavirus (2019-nCoV) has been diagnosed in with an outbreak originating in Wuhan, China[5].
Starting early February 2020, 59 airline companies suspended or limited flights to Mainland China; several countries including the USA, Russia, Australia, and Italy, have also imposed government-issued travel restrictions[6]. As of March 19, 2020, the epidemic situation in China has been effectively restrained. However, the novel coronavirus (2019-nCoV) has spread internationally on a large scale. In response to the current epidemic, Italy has also taken measures to close the city to prevent the large-scale spread of the virus.
The COVID-19 is currently spreading widely in the United States, and the activity levels of new coronaviruses in different regions are various. As it’s reported on by CDC, as of March 20, 2020, 10442 cases have been diagnosed. The outbreaks of the virus in Washington, New York, and California are particularly severe. The U.S. government has adopted a series of unprecedented measures in the areas of travel, medical care, and so on in response to the growing public health threat posed by the novel coronavirus. For example, foreign citizens who have visited China and other 25 countries in the past 14 days are denied entry into the United States, and all elective ambulatory provider visits are delayed.
Features of the Novel Coronavirus
2019-nCoV belongs to the beta coronavirus that genetic characteristics have diversity compared with the Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) and the Middle East Respiratory Syndrome Coronavirus (MERS-CoV)[7]. SARS-CoV[8] and MERS-CoV[9] led to more than 10,000 cases in the past two decades, with a mortality rate of 10% in the case of SARS-CoV and 37% in the case of MERS-CoV[10]. In the case of 2019-nCoV, the number of laboratory-confirmed cases still is rising with a reported mortality rate of around 3%[11].
Thousands of coronaviruses that derived from diverse animals in different regions were sequenced. The results show that protein from 2019-nCoV has more than 85% homology compared with bat SARS coronavirus, and the Wuhan Virus Research team confirmed that 2019-nCoV entered cells by binding to Angiotensin-Converting Enzyme 2 cell receptor, which possibly explains its capacity that crosses the species barrier and infects humans [12, 13]. It takes approximately 6 days to isolate and replicate 2019-nCoV in Vero E6 and Huh-7 cell lines. As for primary cells, human respiratory epithelial cells are suitable for research as its susceptible to 2019-nCoV[14].
Epidemiological Characteristics of Novel Coronavirus
Patients with this new type of coronavirus pneumonia are regarded as the principal source of infection, and asymptomatic infection may also become the source[14]. On February 9, 2020, Guan et al. collected 1,099 patients diagnosed with COVID-19 data from the laboratories of 552 hospitals in China as of January 29, 2020. They found that of the 1099 patients with new coronary pneumonia, 43.95% were local, and the remaining 27.85% had contact with Wuhan personnel, 26% of patients have not traveled to Wuhan recently. These results indicate the presence of familial clustered and asymptomatic infections spread[15].
Laboratory Inspection
The total number of white blood cells in the peripheral blood is regular or decreased, and the lymphocyte count is reduced in the early stage of the onset. The increased liver enzymes, lactate dehydrogenase (LDH), muscle enzymes, or myoglobin are also found in some patients. The increasing troponin can be a crucial index for detecting critically ill patients. The elevated C-reactive protein (CRP) and erythrocyte sedimentation are mostly found in patients.COVID-19 nucleic acid can be detected using RT-PCR technology[16] in specimens such as nasopharyngeal swabs, sputum, lower respiratory tract secretions, blood, and feces[14].
Chest CT Images Features
The early manifestations are mainly sub-pleural patchy ground-glass shadows [17]. As shown in figure 1, the chest CT scan was evaluated for the following characteristics for each patient: (1) presence of ground-glass opacities, (2) presence of consolidation, (3) laterality of ground-glass opacities and consolidation and so on [18, 19]. The lungs of severe patients showed diffuse consolidation. Pulmonary lesions are absorbed, and fibrous foci are formed during recovery[5].
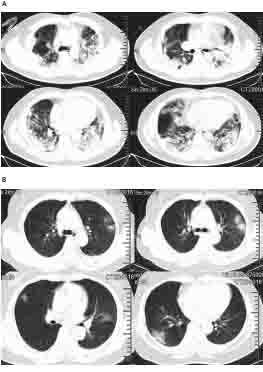
Figure1. Chest CT images. Transverse chest CT images from a 40-year-old man showing bilateral multiple lobular and sub-segmental areas of consolidation on day 15 after symptom onset (A).
Transverse chest CT images from a 53-year-old woman showing bilateral ground-glass opacity and sub-segmental areas of consolidation on day 8 after symptom onset (B) [5].
The 2019-nCoV shows an influential transmission. From the current case data, the minimum reported age is a newborn at 30h, and the maximum reported age is an elderly person over 90 years old. Therefore, the population is generally susceptible[20].
Clinical Typing of Novel Coronaviruses
Patients are divided into 4 types according to the severity of the disease. Light patients have mild clinical symptoms and no pneumonia on imaging. Typical types have symptoms such as fever and respiratory tract. The heavy type meets any of the following: respiratory distress, RR ≥ 30times/min and resting-state mean oxygen saturation ≤ 93%; critically ill those who meet one of the following conditions can be determined as critical: Respiratory failure occurs and requires mechanical ventilation, shock occurs and combined with other organ failures, ICU monitoring and treatment is needed. Doctors will treat patients according to these classification criteria[14]. As shown in figure2, the proportion of mild and asymptomatic cases versus severe and fatal cases is currently unknown for 2019-nCoV that complicates the outbreak response.

Figure2. Surveillance Pyramid and Its Relation to Outbreak Containment[10].
Treatment of 2019-nCoV
Suspected and confirmed cases should be isolated and treated at designated hospitals with effective isolation and protective conditions. Suspected cases should be treated in isolation in a single person. Critical cases should be admitted to the ICU as soon as possible[21]. Unfortunately, no specific coronavirus antivirals or vaccines have been proven to be effective. Remdesivir, a 1′-cyano-substituted adenosine nucleotide analog prodrug with broad-spectrum antiviral activity against several RNA viruses, may be evaluated [22]. In the United States, the first reported patient with COVID-19 infection received treatment with Remdesivir. Remdesivir was given intravenously on the 11th day of illness due to the worsening clinical condition [23]. The drug is currently being used in clinical trials in China, and it is unclear whether it can be used for the diagnosis and treatment of novel coronaviruses. Meanwhile, some national research institutions and pharmaceutical companies announcing the research of the vaccine for the 2019-nCoV is being in pipeline.
Conclusion
In short, in the 2019-nCoV epidemic, China and the international community have responded faster than previous SARS-nCoV. These responses include disease diagnosis, virus isolation, financial support, and temporary hospital construction. However, the normal activities of people are still affected by the spread of the virus.
People need to work hard to reduce panic and economic losses. The current goal is to break the COVID-19 transmission chain, which will require effective procedures to track, diagnose, and cure every infected patient. Besides, we all need to be aware of the risk that zoonotic virus crossing species may infect humans in the future and refuse to eat wild species. We must call for global action to respond to this significant public health emergency.
AcceGen is providing tools that help for fighting against the 2019-nCoV outbreak, include virus RNA/DNA detection kits and cell lines for future diagnostics and therapeutics. For inquiries about the products, please contact inquiry@accegen.com. We wish our resources could support related research to move forward.
References
1. Isaac I. Bogoch M, 2 Alexander Watts, PhD3,4 Andrea Thomas-Bachli, PhD3,4: Potential for global spread of a novel coronavirus from China. 2020.
2. Tang JW, Tambyah PA, Hui DSC: Emergence of a novel coronavirus causing respiratory illness from Wuhan, China. J Infect 2020, 80(3):350-371.
3. Organization WH: Home care for patients with suspected novel coronavirus (nCoV) infection presenting with mild symptoms and management of contacts. https://www.who.int/publications-detail/home-care-for-patients-with-suspected-novel-coronavirus-(ncov)-infection-presenting-with-mild-symptoms-and-management-of-contacts 2020.
4. Luo GG, Gao SJ: Global health concerns stirred by emerging viral infections. J Med Virol 2020, 92(4):399-400.
5. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X et al: Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet 2020, 395(10223):497-506.
6. Chinazzi1 M, JTD, MA, Corrado Gioannini3, Maria Litvinova3, Stefano Merler2, Ana , Piontti1 Py, KM, LR, Kaiyuan Sun4, Cécile Viboud4, Xinyue Xiong1, HY, M, Elizabeth Halloran6, Ira M. Longini Jr.8*, Alessandro Vespignani1,3*: The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science 2020.
7. Ena J, Wenzel RP: A novel coronavirus emerges. Revista Clínica Española (English Edition) 2020, 220(2):115-116.
8. Thomas G. Ksiazek DVM, Ph.D., Dean Erdman, Dr.P.H., Cynthia S. Goldsmith, M.S., Sherif R. Zaki, M.D., Ph.D.,, Teresa Peret PD, Shannon Emery, B.S., Suxiang Tong, Ph.D., Carlo Urbani, M.D.,* James A. Comer, Ph.D., M.P.H., Wilina Lim, M.D., Pierre E. Rollin, M.D., Scott F. Dowell, M.D., M.P.H., Ai-Ee Ling, M.D., Charles D. Humphrey, Ph.D., Wun-Ju Shieh, M.D., Ph.D., Jeannette Guarner, M.D., Christopher D. Paddock, M.D., M.P.H.T.M., Paul Rota, Ph.D., Barry Fields, Ph.D., Joseph DeRisi, Ph.D., Jyh-Yuan Yang, Ph.D., Nancy Cox, Ph.D., James M. Hughes, M.D., James W. LeDuc, Ph.D., William J. Bellini, Ph.D., Larry J. Anderson, M.D., and the SARS Working Group: A Novel Coronavirus Associated with Severe Acute Respiratory Syndrome. N Engl J Med 2003.
9. Assiri A, McGeer A, Perl TM, Price CS, Al Rabeeah AA, Cummings DA, Alabdullatif ZN, Assad M, Almulhim A, Makhdoom H et al: Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med 2013, 369(5):407-416.
10. Vincent J. Munster PD, Marion Koopmans, D.V.M., Neeltje van Doremalen, Ph.D., Debby van Riel, Ph.D., and Emmie de Wit, Ph.D.: A Novel Coronavirus Emerging in China — Key Questions for Impact Assessment. perspective 2020.
11. Jiang S, Xia S, Ying T, Lu L: A novel coronavirus (2019-nCoV) causing pneumonia-associated respiratory syndrome. Cell Mol Immunol 2020.
12. Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L et al: Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. BioRxiv 2020.
13. Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, Zhong W, Hao P: Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci 2020, 63(3):457-460.
14. 国家卫生健康委员会: 新型冠状病毒肺炎诊疗方案(试行第五版修正版). http://www.gov.cn/zhengce/zhengceku/2020-02/09/content_5476407htm 2020.
15. Guan W-j, Ni Z-y, Hu Y, Liang W-h, Ou C-q, He J-x, Liu L, Shan H, Lei C-l, Hui DSC et al: Clinical characteristics of 2019 novel coronavirus infection in China. Medrxiv 2020.
16. Chu DKW, Pan Y, Cheng SMS, Hui KPY, Krishnan P, Liu Y, Ng DYM, Wan CKC, Yang P, Wang Q et al: Molecular Diagnosis of a Novel Coronavirus (2019-nCoV) Causing an Outbreak of Pneumonia. Clin Chem 2020.
17. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R et al: A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med 2020, 382(8):727-733.
18. Xu X, Yu C, Qu J, Zhang L, Jiang S, Huang D, Chen B, Zhang Z, Guan W, Ling Z et al: Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging 2020.
19. Adam Bernheim,Xueyan Mei,Mingqian Huang,Yang Yang,Michael Chung: For each patient, the chest CT scan was evaluated for the following characteristics. 2020.
20. Zhang Y, Xu J, Li H, Cao B: A Novel Coronavirus (COVID-19) Outbreak: A Call for Action. Chest 2020.
21. Nkengasong J: China’s response to a novel coronavirus stands in stark contrast to the 2002 SARS outbreak response. Nat Med 2020.
22. Mulangu S, Dodd LE, Davey RT, Jr., Tshiani Mbaya O, Proschan M, Mukadi D, Lusakibanza Manzo M, Nzolo D, Tshomba Oloma A, Ibanda A et al: A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics. N Engl J Med 2019, 381(24):2293-2303.
23. Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A et al: First Case of 2019 Novel Coronavirus in the United States. N Engl J Med 2020, 382(10):929-936.
Copyright - Unless otherwise stated all contents of this website are AcceGen™ All Rights Reserved – Full details of the use of materials on this site please refer to AcceGen Editorial Policy – Guest Posts are welcome, by submitting a guest post to AcceGen you are agree to the AcceGen Guest Post Agreement – Any concerns please contact marketing@accegen.com




Like!! I blog quite often and I genuinely thank you for your information. The article has truly peaked my interest.
Thanks for your comment! Hope we could continue to provide the latest science and biology insights for you. Take care!:)