Retrovirus Packaging Service
Retroviruses are highly versatile tools in the field of cell and gene therapy, as they enable stable gene delivery for the potential treatment of genetic disorders and the engineering of therapeutic cells. These viruses integrate their cargo into the host cell’s genome, ensuring long-term and stable expression of the transgene. Retroviral vectors have been successfully employed to correct mutations, generate induced pluripotent stem cells (iPSCs), and develop personalized therapies. With their high transduction efficiency, retroviruses are valuable tools for advancing cell and gene therapy research and development.
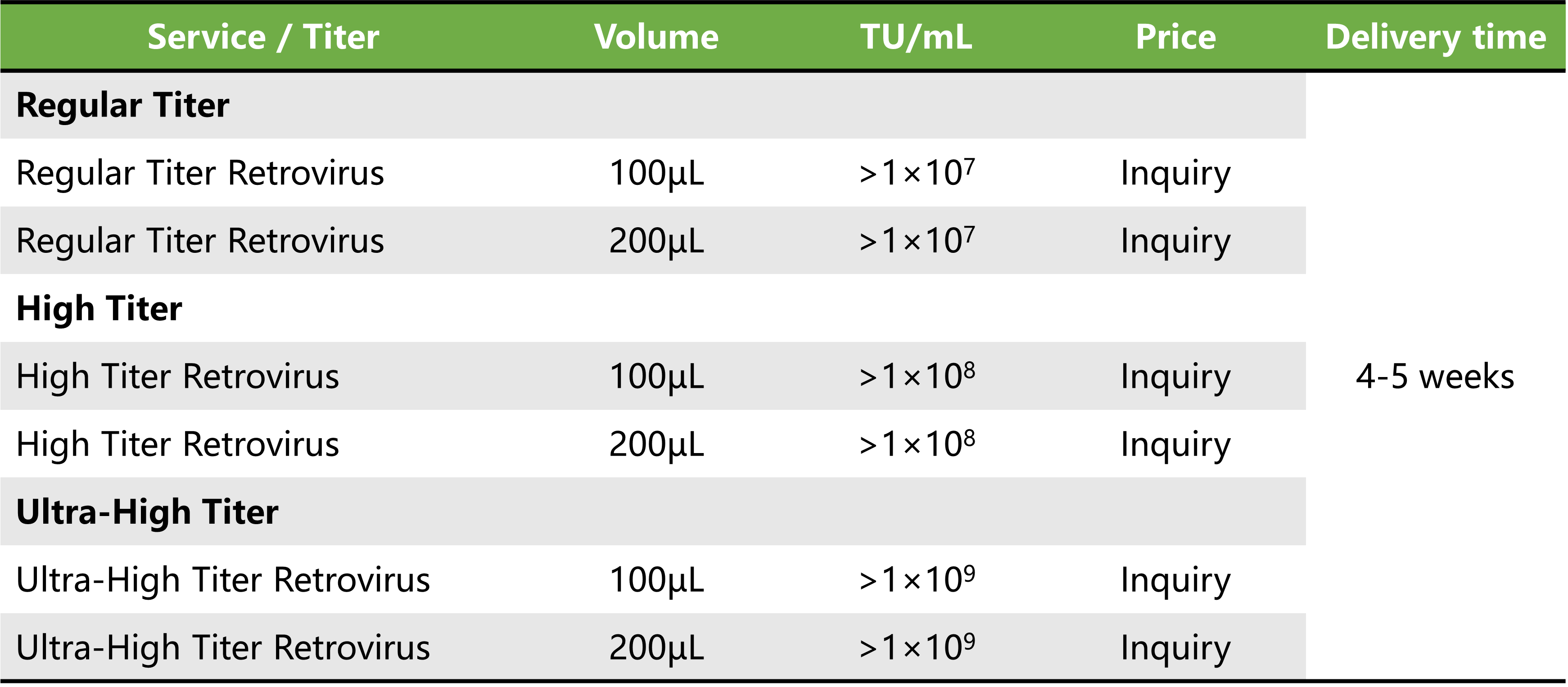
AcceGen offers high-quality retrovirus packaging services for efficient and reliable gene delivery. Our comprehensive service includes rapid turnaround time, rigorous quality control, and accurate titer measurement. We provide ready-to-transduce viral particles, saving you time and effort. By utilize AcceGen’s retrovirus packaging service, you can confidently advance your cell and gene therapy research and applications.
Shipping and storage
Our retrovirus product is stored in HBSS buffer and shipped on dry ice. Upon receiving, it should be stored at -80°C for long-term storage(stable for at least 6 months) or -20°C for use within one week. The shelf life for retrovirus is approximately one year. Please avoid repeated freeze-thaw cycles of retrovirus.
Retrovirus production and quality control (QC)
Our retrovirus manufacturing process involves co-transfection of the transfer vector with proprietary envelope and packaging vectors. Following an appropriate incubation, the supernatant containing virus particles is collected, centrifuged, and filtered. Viral particles are concentrated with polyethylene glycol (PEG). Futhermore, ultra-purified retrovirus undergoes sucrose cushion centrifugation to further enhance its purity. Functional titer is measured using qPCR.
Our quality control includes titer measurement, sterility testing, mycoplasma detection, and optional endotoxin assay. For retrovirus samples that encode fluorescent proteins, we conduct transduction and fluorescence detection tests. Ultra-purified retrovirus samples are routinely tested for virus quality using the endotoxin assay. Additional QC services are available upon request to ensure product quality.
Advantages:
- Efficient gene delivery: Our retrovirus packaging system enables effective transfer of genes into dividing cells for stable expression.
- Versatile applications: Retroviral vectors can deliver genes to various cell types, making them suitable for diverse gene therapy and genetic engineering purposes.
- High-titer production: We ensures the production of high-titer retrovirus particles, maximizing transduction efficiency.
- Quality control: Stringent quality control measures, including titer measurement and sterility testing, guarantee the integrity and safety of retrovirus products.
- Optional ultra-purification: Ultra-purification is available for enhanced retrovirus quality and concentration, optimizing performance in research and therapeutics.
Overall, AcceGen offers efficient retrovirus packaging services for versatile gene delivery, providing high-titer production, rigorous quality control, and optional ultra-purification, ensuring reliable performance in diverse applications.