Stem Cell-derived Exosomes
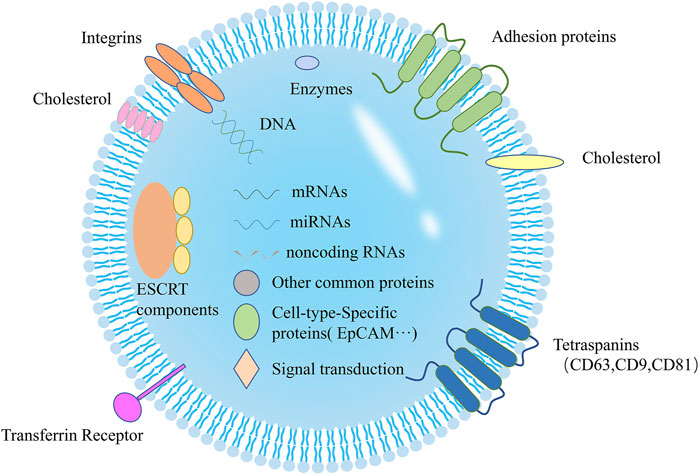
Exosomes are nanoscale extracellular vesicles (EVs) (~50‐150 nm) secreted by various cell types and found in body fluids and cell culture supernatants. Acting as natural messengers, they enable the transfer of proteins, lipids, mRNA, miRNA, and DNA between cells, thereby contributing to intercellular communication. Through this exchange, exosomes influence diverse biological processes such as apoptosis, antigen presentation, angiogenesis, inflammation, coagulation, and the regulation of the tumor microenvironment and immune responses.
In addition, exosomes can be readily obtained from body fluids, and their composition may directly reflect the physiological and/or pathological state of the donor cells. Therefore, they have emerged as powerful tools for disease diagnosis, monitoring, and therapy development. Easily isolated from body fluids, exosomes offer a non-invasive route for early detection of cancer, autoimmune disorders, and other diseases.
Exosome Isolation & Quantification
At AcceGen, multiple technologies are available for isolating and quantifying exosomes, each with its advantages:
- 1. Ultracentrifugation: The gold standard, separating vesicles based on size and density.
- 2. Precipitation: Simple workflow with high yield, though the specificity is intermediate for the difficulty separating exosomes from protein aggregates, lipoproteins, and viruses.
- 3. Immunomagnetic bead method: High specificity, sensitivity, and purity; preserves exosome morphology and supports both qualitative and quantitative analysis.
- 4. Size Exclusion Column (SEC): Excellent for plasma or serum; effectively separates exosomes from important contaminants such as proteins and HDL.
To optimally use SEC for dilute matrices, such as urine or cell culture media, these diluted samples require pre-concentration by ultracentrifugation or ultrafiltration.
- 5. Microfluidics (MF): Microfluidics (MF) technology uses chips with specific antibody-mediated binding to capture exosomes. This antibody-based chip platform offers high-throughput, sensitive, and automated isolation with reduced sample/reagent requirements.
Exosome Characterization and Identification
The exosome characterization is specific and identical, so several characterization indexes are needed to determine whether the extracted components are exosomes. To confirm exosome identity and quality,
AcceGen offers multiple complementary methods, including:
Exosome Isolation & Quantification
- Transmission electron microscope (TEM)
- Scanning electron microscope (SEM)
- Nanoparticle tracking analysis (NTA)
- Western blot (WB)
- Enzyme-linked immunosorbent assay (ELISA)
- Flow cytometry
- Fluorescence-activated cell sorting (FACS).
- Multiplexing bead arrays.
- Gene expression analysis (mRNA and microRNA).
Exosome engineering
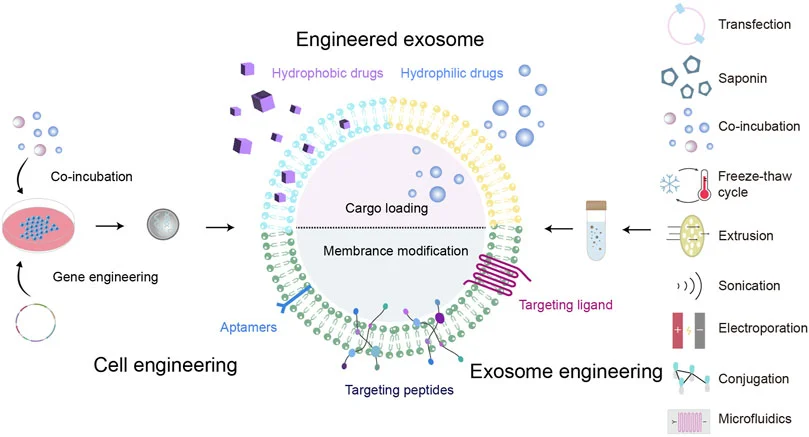
Native exosomes can suffer from poor targeting and rapid clearance in vivo. At AcceGen, to overcome these challenges, we usually modified to form engineered exosomes. The method of gene editing uses genetically modified parent cells as the main strategy to integrate the therapeutic agent into the corresponding exosomes, and is suitable for RNA or proteins that cannot be directly loaded onto exosomes.
Categories:
Cargo Loading – Incorporating therapeutic RNAs, proteins, or small molecules.
Surface Modification – Adding targeting ligands or peptides for enhanced specificity.
Engineered Exosome Production – Gene-editing donor cells to package therapeutic agents directly into secreted exosomes.
Exosome Labeling & Tracking
Exosome fluorescent labeling
Fluorescence exosome purification
Fluorescent exosome concentration labeling
Exosome multiomics analysis
Proteomics: Analysis of exosomal protein expression patterns, functions, and post-translational modifications.
Metabolomics: Identification and quantification of differential metabolites and pathways.
Lipidomics: Detailed analysis of lipid composition and targeted lipid molecules.
Functional Studies
AcceGen assists in conducting functional studies of exosome, including cell-to-cell communication, signal transduction, immune modulation, targeted drug delivery, cell proliferation and disease diagnosis & treatment.
Functional Studies
Exosomes are central players in biological communication. AcceGen supports functional studies including:
Cell-to-cell communication and signal transduction
Immune modulation
Targeted drug delivery
Cell proliferation regulation
Disease diagnosis and therapeutic evaluation
Exosome in Disease Diagnosis
Exosomes serve as natural, biocompatible carriers with strong potential in diagnostics and therapeutics. Their ability to encapsulate and transfer biomolecules makes them powerful tools for:
Genetic Drugs – delivery of siRNA, mRNA, CRISPR components
Liquid Biopsy – non-invasive detection of disease biomarkers
Exosome-Based Therapeutic Strategies
Categories of Therapeutic Cargo:
Genetic Drugs (siRNA, mRNA, CRISPR elements)
Traditional Chinese Medicine Compounds
Conventional Western Pharmaceuticals
Methods:
Pre-secretory Drug Loading: achieved through gene editing or co-incubation with progenitor cells
Post-secretory Drug Loading: including co-incubation with isolated exosomes, electroporation, sonication, extrusion, freeze–thaw cycling, or saponin-assisted methods
Surface Modification: via chemical conjugation of targeting peptides, genetic modification of exosomal membranes or progenitor cells, magnetic nanoparticle integration, electrostatic interactions, or post-insertion techniques