- In-Stock Tumor Cell Lines
- Human Orbital Fibroblasts
- Human Microglia
- Human Pulmonary Alveolar Epithelial Cells
- Human Colonic Fibroblasts
- Human Type II Alveolar Epithelial Cells
- Human Valvular Interstitial Cells
- Human Thyroid Epithelial Cells
- C57BL/6 Mouse Dermal Fibroblasts
- Human Alveolar Macrophages
- Human Dermal Fibroblasts, Adult
- Human Lung Fibroblasts, Adult
- Human Retinal Muller Cells
- Human Articular Chondrocytes
- Human Retinal Pigment Epithelial Cells
- Human Pancreatic Islets of Langerhans Cells
- Human Kidney Podocyte Cells
- Human Renal Proximal Tubule Cells
Chinese Hamster Ovary cells
Chinese Hamster Ovary (CHO) cells were initially isolated from the ovaries of Chinese hamsters in the 1950s and have since become a predominant platform for the production of therapeutic proteins. The first recombinant therapeutic protein generated using CHO cells was tissue plasminogen activator (tPA), which received approval for clinical application in 1987 [1]. Subsequently, CHO cells have been employed to manufacture a broad array of biologics. Notably, between 2018 and 2022, approximately 89% of therapeutic proteins derived from mammalian systems were produced using CHO cells, underscoring their status as the industry standard [2].
CHO cells are particularly valued for their capability to perform complex post-translational modifications (PTMs)—including glycosylation, disulfide bond formation, and proteolytic processing—that closely resemble those occurring in human cells [3, 4]. Although minor differences in specific PTM patterns and structures exist between CHO and human systems, these can be effectively addressed through glycoengineering strategies [5]. Furthermore, compare to other cell lines for protein production, CHO cells exhibit favorable growth characteristics in high-density suspension culture under chemically defined serum-free conditions and demonstrate high recombinant proteins yields. These attributes collectively establish CHO cells as a robust and cost-effective system for large-scale protein manufacturing.
Given their dominance in therapeutic protein production, CHO cells are increasingly being adopted as a promising host for vaccine antigen production. These vaccines are produced by expressing specific antigens in CHO cells, followed by purification to elicit an immune response in recipients [4]. Among the 17 recombinant vaccines currently approved in the United States, four (23.5%) are produced using CHO cells. It is noteworthy that three out of the four recombinant vaccines approved since 2020 utilize CHO cell-derived antigens, with the remaining one produced in HEK cells which is another mammalian expression system. The four FDA-approved vaccines manufactured in CHO cells target Herpes Zoster, Hepatitis B, and Respiratory Syncytial Virus (RSV) infections—with two of these directed against RSV.
FDA-Approved CHO-Derived Vaccines
SHINGRIX (Zoster Vaccine Recombinant, Adjuvanted)
SHINGRIX is manufactured using recombinant CHO cells cultured in serum-free medium to express glycoprotein E (gE) of the varicella-zoster virus. The purified antigen is formulated with an adjuvant system to enhance immunogenicity.
This vaccine is approved for the prevention of herpes zoster in adults aged 50 years and older, as well as immunocompromised individuals aged 18 years and above (e.g., transplant recipients or patients with hematologic malignancies). Administered as two intramuscular doses of 0.5 mL each, SHINGRIX demonstrated 97.2% efficacy in adults over 50 and 68.2% efficacy in immunocompromised hematopoietic stem cell transplant recipients. Protection against shingles and postherpetic neuralgia is sustained for at least 11 years [6, 7].
PREHEVBRIO [Hepatitis B Vaccine (Recombinant)]
PREHEVBRIO is produced via recombinant expression in genetically engineered CHO cells cultured in a nutrient-rich medium. The cells co-express three forms of hepatitis B surface antigens—small (S), middle (pre-S2), and large (pre-S1)—which are subsequently co-purified from the culture supernatant through a series of physicochemical purification steps. These antigens spontaneously assemble into non-infectious virus-like particles (VLPs) that incorporate host-derived membrane lipids [8].
Indicated for the prevention of hepatitis B virus (HBV) infection in adults aged 18 years and older, PREHEVBRIO induces protective antibodies against all known HBV subtypes. Clinical trials demonstrated a seroprotection rate of 91.4% four weeks after the third dose, which is significantly higher than the 76.5% observed with the yeast-derived Engerix-B vaccine. This vaccine addresses a critical unmet need in older adults and high-risk populations, reducing the long-term risks of chronic hepatitis, cirrhosis, and hepatocellular carcinoma [8, 9].
ABRYSVO (Respiratory Syncytial Virus Vaccine)
ABRYSVO is produced using recombinant CHO cells cultured in serum-free conditions to express prefusion F (preF) glycoproteins from RSV subtypes A and B. The antigens are purified via chromatographic methods and formulated with stabilizers—tromethamine, sucrose, and mannitol—prior to lyophilization.
ABRYSVO is indicated for active immunization against RSV-associated lower respiratory tract disease (LRTD) in three populations: pregnant individuals (32–36 weeks gestational age), adults ≥60 years, and high-risk adults aged 18–59 years. Efficacy against severe LRTD in infants within 90 days after birth was 81.8%, and 69.4% through 6 months. In older adults, efficacy was 66.7% against moderate LRTD and 85.7% against severe disease. High-risk younger adults also mounted protective immune responses [10].
AREXVY (Respiratory Syncytial Virus Vaccine, Adjuvanted)
AREXVY is formulated as a two-component vaccine. The antigen component consists of a recombinant RSV PreF3 glycoprotein engineered to maintain the pre-fusion conformation of the RSV F protein. This is produced in genetically modified CHO cells cultured in antibiotic-free, animal-derived protein-free medium.
AREXVY is approved for the prevention of RSV-induced LRTD in adults aged 60 years and older, and in adults aged 50–59 with elevated risk due to underlying conditions such as chronic pulmonary disease, chronic cardiovascular disease, diabetes, or chronic kidney/liver impairment. Clinical studies reported 82.6% efficacy in reducing RSV-LRTD risk during the first RSV season in adults ≥60 years, with 62.9% efficacy maintained over three seasons [11, 12].
Global Research Initiatives on CHO Cell–Based Vaccines
In Europe, CHO cells are widely employed in both academic and industrial vaccine development. For example, the recombinant hepatitis B vaccine GenHevac® B, developed by the Pasteur Institute, exemplifies the successful application of CHO cell technology [13]. Another vaccine manufactured using CHO cells in Europe is Bimervax, which is a COVID-19 vaccine containing recombinant SARS-CoV-2 spike (S) protein receptorbinding domain (RBD) fusion heterodimer. This vaccine is approved also in the EU [14]. Current research focuses on enhancing production efficiency, improving antigen stability, and optimizing cell culture processes. Genetic and metabolic engineering strategies are being applied to increase recombinant protein yield and reduce manufacturing costs [15]. Additionally, GlaxoSmithKline has developed a CHO-derived pentameric antigen complex from human cytomegalovirus (CMV), which elicits neutralizing antibodies in animal models and represents a promising vaccine candidate for clinical development [16].
In the United States, multiple research initiatives are leveraging CHO cells for novel vaccine development. For instance, efforts to produce a subunit vaccine against Zika virus involve engineering CHO cells to express VLPs, capitalizing on their ability to perform human-like glycosylation for improved immunogenicity [17]. Similarly, CHO cells are being used to generate VLP-based vaccines against Nipah virus, which mimic the native virion structure to stimulate immune system without causing actual infection, offering a safe and immunogenic vaccine strategy [18].
In China, several biotechnology firms are advancing CHO-based vaccine candidates. In 2024, Chengdu Maxvax Biotechnology Co., Ltd. initiated phase I/II clinical trials for a novel CHO-based recombinant RSV vaccine employing a proprietary MA103 adjuvant system designed to enhance immunogenicity [19]. Moreover, Jiangsu Zhonghui Yuantong Biotechnology Co., Ltd., along with its subsidiaries, have also developed an adjuvant-formulated recombinant RSV vaccine using CHO cells, which has recently received the approval for IND application from both China and the United States. This vaccine incorporates a modified pre-F antigen conformation, demonstrating higher pre-F expression levels, better thermal stability, and superior immunogenicity compared to already marketed recombinant RSV vaccines [20].
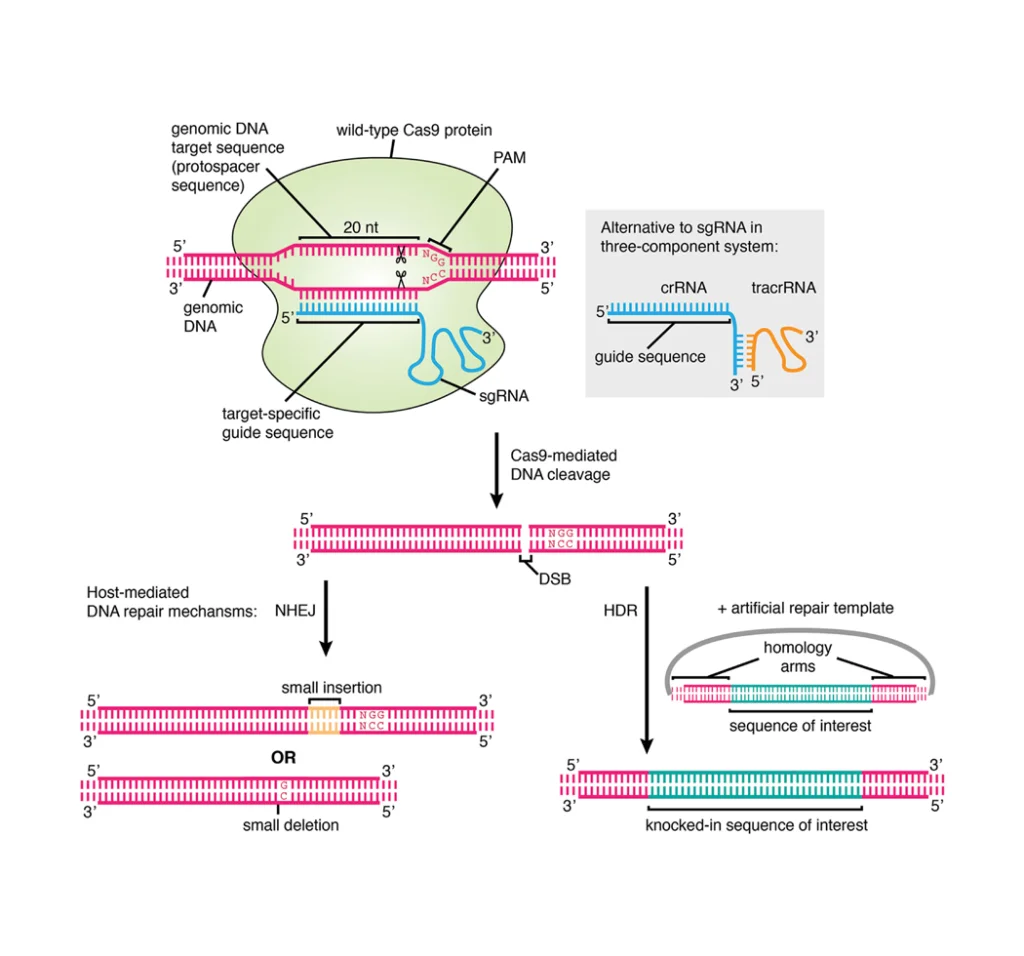
Further research is directed toward optimizing CHO cell culture conditions through the development of serum-free media formulations that support high cell density, viability, and recombinant protein expression [21]. Genetic engineering approaches are also being used to humanize glycosylation patterns of vaccine antigens, thereby improving their safety and efficacy profiles [22]. Figure 1 illustrates current genome editing techniques applied to CHO cells, including four key approaches: gene overexpression, gene knock-out, RNAi-mediated gene knock-down, and miRNA regulation [23].

Figure 1. Cellular functions targeted by genetic engineering in CHO cells.
Limitations
Despite their advantages, there are several limitations associated with the use of CHO cells in vaccine production. A significant challenge is the inherent difficulty in synthesizing and secreting highly complex proteins, which can limit yield and impede large-scale production [24]. Additionally, genetic heterogeneity within CHO cell populations may also lead to batch-to-batch variability, affecting product consistency [25]. Furthermore, CHO cells are susceptible to apoptosis under stress conditions, which can curtain culture longevity and recombinant protein titers [26]. Moreover, as non-human cells, CHO platforms may introduce non-human glycoforms that could potentially affect immunogenicity or biological activity of vaccine antigens. Meanwhile, although CHO cells proliferate rapidly in comparison to other mammalian cell types including HEK cells [27], they exhibit lower growth rates and maximal viable cell densities compared to microbial and yeast based culture systems, complicating process scalability [28]. Despite these challenges, ongoing advances in cell engineering and process development continue to mitigate these limitations, reaffirming the utility of CHO cells in biopharmaceutical applications.
Conclusion
CHO cells continue to serve as a cornerstone of biological vaccine manufacturing, underpinning the production of multiple FDA-approved vaccines with demonstrated clinical efficacy. Ongoing global research efforts across Europe, the United States, and China are expanding their application to new targets, including emerging infectious diseases. Although limitations such as suboptimal growth rates and genetic instability persist, continuous improvements in culture media, genetic engineering, and process control are enhancing the performance and reliability of CHO-based systems. Consequently, CHO cells are poised to remain integral to the development of safe, effective, and scalable vaccines for foreseeable future.
References
[1] K.P. Jayapal, K.F. Wlaschin, W.S. Hu, M.G.S. Yap, Recombinant Protein Therapeutics from CHO Cells – 20 Years and Counting, Chemical Engineering Progress 103(10) (2007) 40-47.
[2] G. Walsh, E. Walsh, Biopharmaceutical benchmarks 2022, Nature biotechnology 40(12) (2022) 1722-1760.
[3] L. Bryan, M. Clynes, P. Meleady, The emerging role of cellular post-translational modifications in modulating growth and productivity of recombinant Chinese hamster ovary cells, Biotechnology advances 49 (2021) 107757.
[4] R. Haryadi, K.F. Chan, P.C. Lin, Y.L. Tan, C. Wan, W. Shahreel, S.J. Tay, T. Nguyen-Khuong, I. Walsh, Z. Song, Generating and characterizing a comprehensive panel of CHO cells glycosylation mutants for advancing glycobiology and biotechnology research, Scientific reports 14(1) (2024) 23068.
[5] Z.V. Sanchez-Martinez, S.P. Alpuche-Lazcano, M. Stuible, Y. Durocher, CHO cells for virus-like particle and subunit vaccine manufacturing, Vaccine 42(10) (2024) 2530-2542.
[6] SHINGRIX | FDA, (n.d.). 2025. https://www.fda.gov/vaccines-blood-biologics/vaccines/shingrix (accessed August 19, 2025).
[7] A. Strezova, J. Díez Domingo, A.L. Cunningham, T. Eto, C. Andrews, C. Arns, E.J. Choo, D.S.C. Hui, G. Icardi, S.A. McNeil, A. Põder, P. Kosina, L. Rombo, T.F. Schwarz, J.C. Tinoco, C.J. Yu, J. Wang, J. Soni, M. Tsang, R. Leon, A. Mwakingwe-Omari, Final analysis of the ZOE-LTFU trial to 11 years post-vaccination: efficacy of the adjuvanted recombinant zoster vaccine against herpes zoster and related complications, EClinicalMedicine 83 (2025) 103241.
[8] PREHEVBRIO | FDA, (n.d.), 2025. https://www.fda.gov/vaccines-blood-biologics/prehevbrio.
[9] F. Diaz-Mitoma, Safety & immunogenicity of a 3-Antigen hepatitis B vaccine, PreHevbrio™ [Hepatitis B vaccine (recombinant)], (2022).
[10] ABRYSVO | FDA, (n.d.). 2025. https://www.fda.gov/vaccines-blood-biologics/abrysvo (accessed August 20, 2025).
[11] AREXVY | FDA, (n.d.). 2025. https://www.fda.gov/vaccines-blood-biologics/arexvy (accessed August 20, 2025).
[12] J. Jiang, H. Hu, L. Cao, N. Mao, Z. Zhu, N. Wang, Y. Shi, H. Li, Y. Zhang, Comparative Evaluation of Three Nanoparticle Vaccines Targeting the Prefusion F Protein of Respiratory Syncytial Virus: Immunogenicity and Protective Efficacy, Int J Nanomedicine 20 (2025) 9945-9961.
[13] J.K. Ho, B. Jeevan-Raj, H.J. Netter, Hepatitis B Virus (HBV) Subviral Particles as Protective Vaccines and Vaccine Platforms, Viruses 12(2) (2020) 126.
[14] European Medicines Agency. Bimervax authorization details. EMA Medicines Database., 2023. https://www.ema.europa.eu/en/medicines.
[15] CHO Cell Line in Bioproduction: Applications and Innovations, (n.d.). 2025. https://www.cytion.com/Knowledge-Hub/Cell-Line-Insights/CHO-Cell-Line-in-Bioproduction-Applications-and-Innovations/ (accessed August 20, 2025).
[16] I. Hofmann, Y. Wen, C. Ciferri, A. Schulze, V. Fühner, M. Leong, A. Gerber, R. Gerrein, A. Nandi, A.E. Lilja, A. Carfi, H. Laux, Expression of the human cytomegalovirus pentamer complex for vaccine use in a CHO system, Biotechnology and bioengineering 112(12) (2015) 2505-2515.
[17] K.A. Dowd, M. Schroeder, E. Sanchez, B. Brumbaugh, B.M. Foreman, K.E. Burgomaster, W. Shi, L. Wang, N. Caputo, D.N. Gordon, C.L. Schwartz, B.T. Hansen, M. Aleshnick, W.P. Kong, K.M. Morabito, H.D. Hickman, B.S. Graham, E.R. Fischer, T.C. Pierson, pr-independent biogenesis of infectious mature Zika virus particles, bioRxiv : the preprint server for biology (2024).
[18] B.B. Larsen, T. McMahon, J.T. Brown, Z. Wang, C.E. Radford, J.E. Crowe, Jr., D. Veesler, J.D. Bloom, Functional and antigenic landscape of the Nipah virus receptor-binding protein, Cell 188(9) (2025) 2480-2494.e22.
[19] Maxvax Launches Clinical Trials for RSV Vaccine, 2025. https://en.maxvax.cn/info.aspx?t=9&id=111.
[20] Zhonghui Biotech’s CHO Cell New Drug Clinical Trial Approved by Chinese and American Authorities – Futubull, (n.d.). 2025. https://news.futunn.com/en/flash/19252107/zhonghui-biotech-s-cho-cell-new-drug-clinical-trial-approved?level=1&data_ticket=1755676434303979 (accessed August 20, 2025).
[21] W. Li, Z. Fan, Y. Lin, T.Y. Wang, Serum-Free Medium for Recombinant Protein Expression in Chinese Hamster Ovary Cells, Frontiers in bioengineering and biotechnology 9 (2021) 646363.
[22] S.W. Li, M. Wright, J.F. Healey, J.M. Hutchinson, S. O’Rourke, K.A. Mesa, P. Lollar, P.W. Berman, Gene editing in CHO cells to prevent proteolysis and enhance glycosylation: Production of HIV envelope proteins as vaccine immunogens, PloS one 15(5) (2020) e0233866.
[23] S. Fischer, R. Handrick, K. Otte, The art of CHO cell engineering: A comprehensive retrospect and future perspectives, Biotechnology advances 33(8) (2015) 1878-96.
[24] Cell-Line Discussions at BPI West 2024, (n.d.). 2024. https://www.bioprocessintl.com/cell-line-development/cell-line-discussions-at-bpi-west-2024 (accessed August 20, 2025).
[25] M.J. Wurm, F.M. Wurm, Naming CHO cells for bio-manufacturing: Genome plasticity and variant phenotypes of cell populations in bioreactors question the relevance of old names, Biotechnology journal 16(7) (2021) e2100165.
[26] C.A. Orellana, V.S. Martínez, M.A. MacDonald, M.N. Henry, M. Gillard, P.P. Gray, L.K. Nielsen, S. Mahler, E. Marcellin, ‘Omics driven discoveries of gene targets for apoptosis attenuation in CHO cells, Biotechnology and bioengineering 118(1) (2021) 481-490.
[27] L. Abaandou, D. Quan, J. Shiloach, Affecting HEK293 Cell Growth and Production Performance by Modifying the Expression of Specific Genes, Cells 10(7) (2021).
[28] F.M. Wurm, Production of recombinant protein therapeutics in cultivated mammalian cells, Nature biotechnology 22(11) (2004) 1393-8.

Copyright - Unless otherwise stated all contents of this website are AcceGen™ All Rights Reserved – Full details of the use of materials on this site please refer to AcceGen Editorial Policy – Guest Posts are welcome, by submitting a guest post to AcceGen you are agree to the AcceGen Guest Post Agreement – Any concerns please contact marketing@accegen.com