- In-Stock Tumor Cell Lines
- Human Orbital Fibroblasts
- Human Microglia
- Human Pulmonary Alveolar Epithelial Cells
- Human Colonic Fibroblasts
- Human Type II Alveolar Epithelial Cells
- Human Valvular Interstitial Cells
- Human Thyroid Epithelial Cells
- C57BL/6 Mouse Dermal Fibroblasts
- Human Alveolar Macrophages
- Human Dermal Fibroblasts, Adult
- Human Lung Fibroblasts, Adult
- Human Retinal Muller Cells
- Human Articular Chondrocytes
- Human Retinal Pigment Epithelial Cells
- Human Pancreatic Islets of Langerhans Cells
- Human Kidney Podocyte Cells
- Human Renal Proximal Tubule Cells
Human Peripheral Blood Mononuclear Cells
Human peripheral blood mononuclear cells (PBMCs) are collected for a variety of research and clinical applications, primarily because they provide a valuable resource for understanding the human immune system. PBMCs include T and B lymphocytes, natural killer (NK) cells, monocytes and dendritic cells. The frequencies of these populations vary across individuals. In general, lymphocytes are in the range of 70-90%, monocytes are from 10-20%, while DCs are rare (only 1-2%). The subpopulation of lymphocytes is 70-85% CD3+ T cells, 5-10% B cells and 5-20% NK cells. CD3+ lymphocytes are composed of CD4+ and CD8+ T cells, roughly in 2:1 ratio. Activated CD4+ T cells may develop into diverse effector cell subsets, including Th1, Th2, Th9, Th17, Th22, follicular helper (Tfh) cells and different types of regulatory cells [1]. The CD4+ helper T cells are essential mediators of immune homeostasis and inflammation [2].
The method to prepare PBMCs is critically important because it directly impacts the viability, purity, functionality, and reproducibility of the cells used in downstream applications including flow cytometry, RNA sequencing, ELISpot/ELISA, vaccine response studies, immune profiling, as well as in vitro /in vivo function assays. Key factors in PBMC preparation includes 1). Blood collection method (anticoagulant used); 2). Time from collection to processing; 3). Density gradient medium (e.g., Ficoll, Histopaque); 4). Centrifugation speed and time; 5). Washing and storage conditions; 6). Procedure under sterile conditions [3].
Materials, Reagents and Instruments
Materials
Personal PPE
Vacutainer® tubes with anticoagulant (or EDTA‐coated vials/tubes)
Tourniquet
Exel International Blood Collecting Sets (with 12” tubing with either 21g x ¾”, 23g x ¾”, or 25g x ¾” needles).
15 mL and 50 mL conical tubes
Serological pipettes (5 mL, 10 mL, 25 mL)
Pipette tips (sterile, filtered)
Sterile 1.5 mL or 2 mL microcentrifuge tubes
Cell strainer (optional)
Freezing container (vials)
Reagents
Ficoll-Paque™ or Histopaque-1077
Phosphate-buffered saline (PBS) (without calcium and magnesium)
RPMI 1640 (or other suitable cell culture medium)
Fetal bovine serum (FBS)
Trypan Blue (optional)
DMSO + FBS (e.g., 10% DMSO in FBS)
Red blood cell (RBC) lysing solution (optional)
Gibco™ Recovery™ Cell Culture Freezing Medium or Freezing medium containing 50% RPMI, 40% FBS, and 10% DMSO
Instruments
Centrifuge with swing-out rotor
Biological safety cabinet (Class II)
Refrigerated centrifuge (optional but ideal)
Hemocytometer or automated cell counter
Water bath at 37 °C (if needed for warming media)
-80 °C freezer
Cryogenic dewars
Procedure for PBMCs Preparation and Cryopreservation
1. Warm Ficoll density gradient medium to room temperature.
2. Prepare PBS wash buffer by adding 2% HI-FBS to 1X sterile Ca2+/Mg2+free PBS.
3. Using aseptic technique, transfer anticoagulant-treated blood from each blood collection tube (or buffy coat unit) into 50 mL sterile conical tubes.
4. Dilute blood with an equal volume of PBS wash buffer (1:1 dilution).
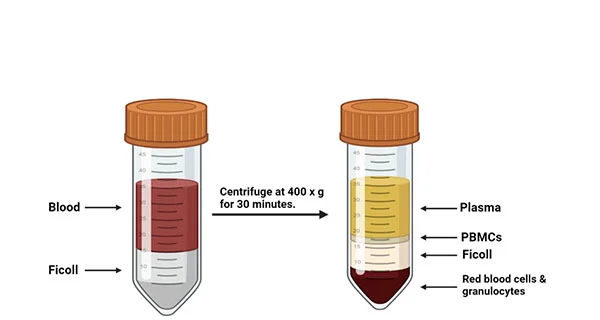
5. Slowly layer 20 mL diluted blood on top of the Ficoll without mixing (Tip: Hold the tube at a 45°angle and let blood slide down the wall).
6. Centrifuge at 400 × g for 30 minutes at room temperature, brake OFF (This step separates the PBMC layer at the plasma-Ficoll interface.).
7. Slowly remove the top diluted plasma layer (~15 mL) using a plastic Pasteur pipette or a serological pipette.

Figure 1. A diagram indicating blood sample layered onto Ficoll media (left tube) and the layers prior to be removed after centrifugation (right tube).
8. Carefully collect the PBMC layer using a sterile Pasteur pipette, starting at the periphery, and slowly moving the pipette tip over the entire cross-sectional area of the tube. Avoid aspirating too much Ficoll medium as this can be toxic to the cells.
9. Carefully transfer the white, cloudy buffy coat layer (PBMCs) to a new 50 mL conical tube by using a sterile pipette. Discard the remaining Ficoll and RBC layers.
10. Add PBS (or RPMI) to 50 mL, mix gently.
11. Centrifuge at 300 × g for 10 minutes at room temperature (18 °C to 20 °C) with brake ON.
12. Discard the supernatant.
13. Repeat wash 1–2 more times to remove the platelets and Ficoll.
14. Transfer the PBMCs to a fresh 50 mL conical tube.
15. Wash the PBMCs by bringing up the volume in the conical tube to 50 mL using PBS wash buffer.
16. Centrifuge at 300 x g for 10 minutes at room temperature (18 °C to 20 °C) with the BRAKE ON.
17. Carefully discard the supernatant and loosen the cell pellet in the remaining solution, using a transfer pipette to mix the large clumps.
18. Optional step only if there are RBCs in the PBMCs: If using a 10X stock solution, dilute it to a 1X working concentration by adding 1 part buffer to 9 parts deionized water, and warm up 1X RBC buffer to room temperature before use. Add 9 mL of RBC lysing solution per 1 mL of cell suspension. Mix gently and incubate on ice for 5-10 minutes.
19. Monitor for turbidity; lysis is complete when the sample becomes clear, stop the lysis reaction by adding dPBS wash buffer (optional solution: dPBS with 5-10% FBS) to 50 ml.
20. Centrifuge at 300 x g for 5-10 minutes at room temperature (18 °C to 20 °C) with the BRAKE ON.
21. Carefully remove the supernatant (liquid containing lysed RBCs).
22. Wash the PBMCs with cold dPBS once.
23. Centrifuge at 300 x g for 5-10 minutes at room temperature (18 °C to 20 °C) with the BRAKE ON.
24. Resuspend the cells in an appropriate amount of cold dPBS wash buffer.
25. Count the cells using an automated cell counter for cell viability and density.
26. Repeat step 23.
27. Resuspend the cells in RPMI with 10% FBS for subsequent assays or designated freezing media appropriate for the application (usually at 0.5-10 million cells/ml/vial).
28. Place PBMC-containing cryovials in a freezing container (e.g., Cool Cell, Mr. Frosty) in a -80 °C freezer overnight.
29. Transfer the vials to liquid nitrogen for long-term storage the next day.
30. A post-thaw rest period of 2 to 24 hours is typically recommended to allow PBMCs to recover full functionality before use.
Conclusion
PBMCs are essential tools in immunology, infectious disease research, oncology, and vaccine development. These blood-derived cells offer a representative snapshot of individual immune systems and serve as robust models for investigating immune responses, disease mechanisms, and therapeutic efficacy. AcceGen provides high-quality cryopreserved human PBMCs isolated from thoroughly screened healthy and valuable diseased donors to support reproducible, scalable, and standardized research applications.
References
[1] K. Verhoeckx, P. Cotter, I. López-Expósito, C. Kleiveland, T. Lea, A. Mackie, T. Requena, D. Swiatecka, H. Wichers (Eds.), The Impact of Food Bioactives on Health: in vitro and ex vivo models, Springer. Cham (CH), 2015.
[2] K. Hirahara, A. Poholek, G. Vahedi, A. Laurence, Y. Kanno, J.D. Milner, J.J. O’Shea, Mechanisms underlying helper T-cell plasticity: implications for immune-mediated disease, The Journal of allergy and clinical immunology 131(5) (2013) 1276-87.
[3] H.W. Grievink, T. Luisman, C. Kluft, M. Moerland, K.E. Malone, Comparison of Three Isolation Techniques for Human Peripheral Blood Mononuclear Cells: Cell Recovery and Viability, Population Composition, and Cell Functionality, Biopreservation and biobanking 14(5) (2016) 410-415.

Copyright - Unless otherwise stated all contents of this website are AcceGen™ All Rights Reserved – Full details of the use of materials on this site please refer to AcceGen Editorial Policy – Guest Posts are welcome, by submitting a guest post to AcceGen you are agree to the AcceGen Guest Post Agreement – Any concerns please contact marketing@accegen.com