Abstract
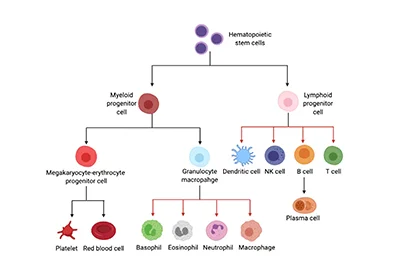
Skeletal Muscles are composed of many contractile muscle cells and are covered by connective tissue. During embryonic and fetal development, muscle stem cells fuse to form muscle fibers. Skeletal Muscle Cells are composed of Skeletal Muscle Stem Cells, Skeletal Muscle Satellite Cells (Skeletal Muscle SCs) , Fibro/Adipogenic Progenitors (FAPs), Macrophages, Neutrophils, Endothelial Cells, C2C12 Skeletal Muscle Myoblast and so on[1]. The functions of different cells in skeletal muscle regeneration were researched.
Product Background
Skeletal muscle is the most important organ of human beings playing an important role in physical activity[2]. Skeletal muscle has a remarkable capacity to regenerate even after repeated traumas, yet limited information is available on muscle repair mechanisms and how they have evolved.
Skeletal regeneration is a very complex biological process in which skeletal muscle stem cells participate in the repair process[3]. Skeletal muscle repair and regeneration rely on driving muscle development to help muscle regeneration.
Muscle Stem Cells (Satellite Cells), Mononuclear Progenitor Cells and Macrophages are the basis of skeletal muscle tissue. Therefore, the studies of skeletal muscle cell types and their respective functions in skeletal muscle regeneration make a major contribution in the treatment of bone diseases.
The Latest Research
-
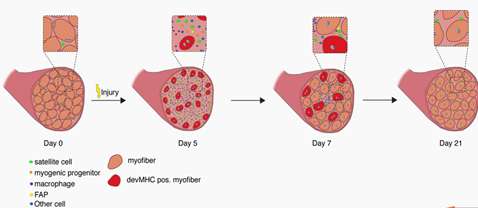
1. Satellite Cells are Considered the Primary Player of Regenerative Myogenesis, Adult Skeletal Muscle Maintains a Relatively Remarkable Ability to Regenerate and Recover Following Mild Damage or Insult throughout Much of the Lifespan.
Application: Skeletal muscle satellite cells do not cause muscle atrophy that suggested its primary function in adults is bone regeneration. The satellite community is considered to be responsible for post-natal muscle growth and tissue regeneration after appropriate stimulation. The research shows TF Yin Yang1 and N-methyltransferase inhibitors can regulate skeletal muscle regeneration through controlling the metabolic reprogramming of satellite cells.