Post Date:Oct-27-21
Brain Microvascular Endothelial Cells Functions and Applications
 Author: AcceGen R&D Team
Author: AcceGen R&D TeamBackground
Brain endothelial cells (BECs) are the major element of the blood-brain barrier (BBB), preventing the brain from pathogens and restricts access to circulating factors. Brain microvascular endothelial cells (BMVECs) form the interface between nervous tissue and circulating blood, comprising primary limitation to passage of toxins and immune cells from the blood into the brain via paracellular, transporter, transcellular, and extracellular matrix proteins.
Compared with peripheral endothelial cells, BMVECs have unique features. For example, they have numerous intercellular “tight junctions” which show high transendothelial electrical resistance and retard paracellular flux; And BMVECs lack fenestrae resulting in a reduced level of fluid-phase endocytosis; In contrast to peripheral ECs, BMVECs contain significantly fewer pinocytotic vesicles and more mitochondria; What’s more, asymmetrically-localized enzymes and carrier-mediated transport systems engender a truly “polarized” phenotype [1,2].
Now, BMVECs are recognized not only as a simple physiological barrier, but also as a highly active metabolic system that synthesizes various materials to nourish nerves and regulate vasomotor function. Such as neurotrophins (BDNF, IGF-1, VEGF) and matrix metalloproteinases (MMPs), which are key components in proteolytic BBB disruption during ischemic stroke, and contribute to vascular edema, hemorrhagic transformation, and leukocyte infiltration [2,3].
In the light of the organ specificity of microvascular endothelial cells, they should be derived from the tissue involved in the diseases researchers wish to study. Most of the BMVECs commonly used in research are human-derived primary cells. Mouse or rabbit BMVECs are less used.
BMVECs Culture Protocol
Take the primary isolation and culture of human BMVECs as example. The procedure is refereed to Bernas, Michael J et al. [4].
1. Reagents
Human brain tissue (can be obtained from discarded tissue during operative treatment of epilepsy (outside of epileptegenic foci), ranging in size from 5–10 mm3 to as large as 4 cm3)
Acetic Acid solution (0.02 N)
Type I Collagen I, rat tail tendon (50 μg/ml in 0.02 N acetic acid for coating of T-25 flask)
DMEM/Ham’s F12
Fetal Bovine Serum (FBS) (10%)
Antibiotic-Antimycotic, stabilized (1%)
Glutamax (1%)
Endothelial Cell Growth Supplement (ECGS) (50μg/ml, 2% for ISM)
Heparin (1 mg/ml, 3.4% for ISM)
(The above reagents can be obtained from AcceGen)
2. Methods
Prior to HBMVEC isolation
1) Coat a T-25 flask using an incubation of 2.5 ml of collagen solution for 1 hour. Wash three times with HBSS.
2)Prepare both Maintain isolation medium (IM) and isolation supplemented medium (ISM).
HBMVEC isolation and culture
3) Maintain IM and ISM at 37ºC in water-bath.
4) Collect the brain sample in a 100 mm tissue culture dish containing 5 ml of IM. To avoid contamination with microorganisms, tissue processing should be performed in a class II biosafety cabinet using sterile reagents.
5) Use sterilized surgical forceps and a dissecting stereomicroscope to carefully remove meninges and visibly large vessels to facilitate the visualization.
6) Use 25, 10 and 5 ml sterile pipettes to repeatedly triturate tissue fragment until the sample can easily pass back and forth through the 5 ml pipette.
7) Place a 500 μm polyester screen (large enough to overlap) over a 100 mm non-coated Petri dish. The membrane is strong enough to not require a wire backing. Wet the screen with 5 ml IM.
8) Pipet the sample onto the polyester screen by starting in the center and working your way out without pushing the sample through the screen. Wash the screen with 10 ml IM. This step eliminates larger non-dissociated tissues which will not pass through the membrane. These tissues may contain some microvascular fragments, but their inclusion in the culture initiation greatly reduces the establishment and purity of the endothelial cultures.
9) Discard the polyester screen and large chunks and preserve the flow through fluid.
10) Place a sterile wire frame on a sterile non-coated 100 mm Petri dish and then place a sterile 30 μm polyester screen on top. Wet the screen with 5 ml IM.
11) Pipet the collected pass-through fluid onto the 30 μm nylon screen. Start in the center and work in a circle and do not try to push sample through the membrane. Wash with 10ml IM.
12) Open another Petri dish and using an additional 10 ml IM in a serological pipet, hold and fold the screen from step 11 and wash the isolated fragments off the membrane into the new non-coated dish. Repeat with 5 ml IM.
13) Transfer the medium containing all microvascular fragments washed off the membrane in Step 12 into a 50 ml conical tube and centrifuge at 400 rpm (30 g) for 10 min.
14) Discard the supernatant and resuspend the pellet in 1 ml ISM.
15) Transfer to a collagen-I coated T-25 flask. Gently shake to ensure coverage of the flask. Allow for attachment of microvessel fragments by placing in the incubator for 1 hour.
16) Carefully introduce an additional 3 ml ISM to the T-25 flask and without shaking, move to the incubator.
Expansion of the HBMVEC culture
17) In 2 to 4 days, change the medium of the culture for the first time with 4 ml ISM. Under phase-contrast microscopy, endothelial cells should begin migrating out from vessels and proliferating. Also monitor for any bacterial or fungal contaminants in the culture.
18) Change the medium once a week. Typically, small endothelial cell clusters are visible after 8-10 days and cell confluency is achieved in 30 days, although this is dependent on the density of seeding. HBMVEC will present as tightly packed triangular shaped cells under phase-contrast microscopy. [4].
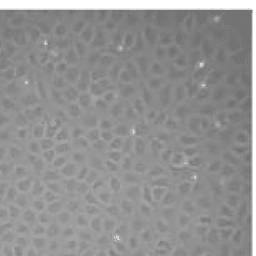
Figure 1. Phase contrast microscopy of human brain microvascular endothelial cells[4]. Scale bar = 100 μm.
Application of BMVECs
BBB is a dynamic and complex interface between central nervous system (CNS) and peripheral blood circulation system. The BBB tightly controls the exchanges between the blood and brain compartments which protects and maintains the delicate interstitial environment that is optimal for neuronal communication. Recent years,in the studying of CNS drug pharmacokinetics, CNS therapeutic targeting, neuroinflammation, neurodegeneration, neuroprotection, and neurotoxicity, the in-vitro BBB model coupled with various technological platforms and co-culture configurations has become an indispensable increasingly important tool.
For example, researchers use the BMVECs to establish in vitro BBB model, to prove the effect of cannabinoid receptor 2 on BBB protection in neuroinflammatory response, provide a platform for drug screening on anti-inflammatory.[5]
BMVECs are also used to study the effects of drugs or toxicants on the permeability of the blood-brain barrier, For example, the regulation and mechanism of MMP3 and MiR-18a on the BBB [6,7].
Future Perspective
BMVECs are the key components of BBB and constitute a physiological barrier between the central nervous system and peripheral blood circulation. More importantly, it is also an active metabolic system that can synthesize various substances to nourish nerves and regulate vasomotor function.
In short, due to its unique characteristics, brain endothelial cells in vitro are widely used in experiments of drug development. Besides, BMVECs are also commonly used in studying mechanism of many neurological diseases (such as stroke) [2]. And its co-cultivation with other nerve cells and the exploration of more ways to obtain them are still very promising areas to many researchers.
Where to Get Brain Microvascular Endothelial Cells for research?
AcceGen provides various types of brain microvascular endothelial cells to meet all your research needs. Human Brain Microvascular Endothelial Cells are AcceGen featured products that backed by AcceGen advanced technology. We also provides animal primary brain endothelial cells, such as C57BL/6 Mouse Brain Microvascular Endothelial Cells, BALB/c Mouse Brain Microvascular Endothelial Cells, and Rat Brain Microvascular Endothelial Cells. More types are available in AcceGen: Brain Microvascular.
It is our pleasure to help relative researches to move forward. All the products of AcceGen are strictly comply with international standards. For more detailed information, please visit our product portfolio or contact inquiry@accegen.com.
Reference
[1] Pong S, Karmacharya R, Sofman M, et al. The Role of Brain Microvascular Endothelial Cell and Blood-Brain Barrier Dysfunction in Schizophrenia[J]. 2020.
[2] Targeting brain microvascular endothelial cells: a therapeutic approach to neuroprotection against stroke[J]. Neural Regeneration Research, 2015, 10(11):1882-1891.
[3] Wang X, Yu X, Chong X , et al. Rescue of Brain Function Using Tunneling Nanotubes Between Neural Stem Cells and Brain Microvascular Endothelial Cells[J]. Molecular Neurobiology, 2015, 53(4):2480-2488.
[4] Bernas M J, Cardoso F L , Daley S K , et al. Establishment of primary cultures of human brain microvascular endothelial cells to provide an in vitro cellular model of the blood-brain barrier[J]. Nature Protocols, 2010, 5(7):1265.
[5] Ramirez S H, Hasko J, Skuba A, et al. Activation of cannabinoid receptor 2 attenuates leukocyte-endothelial cell interactions and blood-brain barrier dysfunction under inflammatory conditions.[J]. Journal of Neuroscience the Official Journal of the Society for Neuroscience, 2012, 32(12):4004-16.
[6] Zhang Q, Zheng M, Betancourt C E , et al. Increase in Blood-Brain Barrier (BBB) Permeability Is Regulated by MMP3 via the ERK Signaling Pathway[J]. Oxidative Medicine and, Cellular Longevity, 2021, 2021(5):1-14.
[7] Ren S, Wu G, Huang Y, et al. MiR-18a Aggravates Intracranial Hemorrhage by Regulating RUNX1-Occludin/ZO-1 Axis to Increase BBB Permeability[J]. Journal of stroke and cerebrovascular diseases: the official journal of National Stroke Association, 30(8):105878.
Copyright - Unless otherwise stated all contents of this website are AcceGen™ All Rights Reserved – Full details of the use of materials on this site please refer to AcceGen Editorial Policy – Guest Posts are welcome, by submitting a guest post to AcceGen you are agree to the AcceGen Guest Post Agreement – Any concerns please contact marketing@accegen.com